Abstract
Background
Aggressive B-cell lymphomas, such as Mantle cell lymphoma (MCL), are microenvironment-dependent tumors but, in contrast to tumoral intrinsic anomalies, complex interplays within their ecosystems are largely ignored. A better understanding of these dialogs could provide new perspectives integrating the key role of the microenvironment to increase treatment efficiency of this hard to cure B-cell malignancy.
Methods
To identify novel molecular regulations occurring in lymphoma protective ecosystems, we performed a transcriptomic analysis based on the comparison of publicly available datasets from circulating (PB, n=77) versus MCL lymph nodes (LN, n=107) together with deep RNA sequencing of purified CD19+CD5+ MCL (n=8) versus normal B-cells (n=6). This integrated analysis led to the discovery of microenvironment-dependent and tumor-specific secretion of the cytokine IL32β by lymphoma cells. Using in situ multiplex immunohistochemistry , ex vivo models of primary MCL cells (n=23) and IL32 KO MCL cell lines (Crispr/Cas9), we studied the regulation and the functional impact of IL32β in the MCL context, especially in the dialog with tumor-associated macrophages.
Results
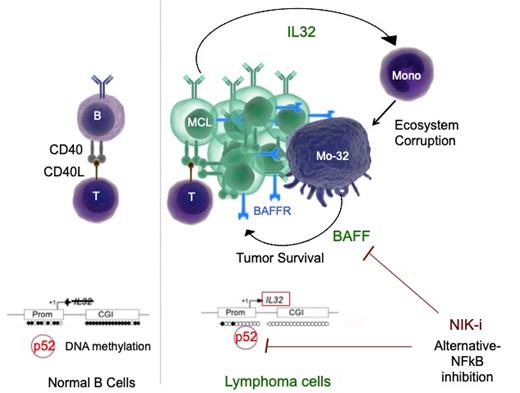
Among the 6887 genes differentially expressed in MCL LN compared to PB in vivo, 70% were confirmed in CD19+ MCL cells cocultured ex vivo and 39% were tumor-specific, that is to say not upregulated in cocultured normal B cells (NBC). Top-genes scoring revealed that IL32 was the most upregulated genes within the "Tumor-specific" transcriptional program. Using ex vivo models of primary MCL cells, we demonstrated that the microenvironment-dependent secretion of IL32β was controlled by the CD40/NFKB2 axis whereas its tumor specificity was the consequence of IL32 promoter hypomethylation in MCL compared to NBC.
IL32 protein expression was confirmed in MCL LN in situ by multiplex IHC. The latter allowed the concurrent detection of MCL cells, T cells, macrophages and IL32. Consistently with the microenvironment-dependent induction of IL32 in MCL, we observed that IL32 expression was enriched in situ in tumor zones infiltrated with T cells, compared to tumor-exclusive zones.
Based on in vitro experiments using IL32 KO MINO cells (Crispr/Cas9), we demonstrated that, through the secretion of IL32β, the tumor was able to corrupt its microenvironment by polarizing monocytes into specific protumoral CD163 mid MCL-associated macrophages, which secrete both pro- (e.g. IL6, OSM, IL1a/b) and anti- inflammatory (e.g. IL10, IDO, IL18, IL4L1) soluble factors. We next highlighted that IL32β-stimulated macrophages supported tumor survival mostly through a soluble dialog, which is driven by BAFF.
Finally, we demonstrated the efficacy of selective NIK/alternative-NFkB inhibition to counteract both microenvironment-dependent induction of IL32β (RNA expression inhibition: 62 % ; n= 3) and BAFF-dependent survival of MCL cells (survival support inhibition : 47 % ; n=6).
Conclusions
In summary, our data uncovered the IL32β/BAFF axis as a previously undescribed pathway involved in MCL-associated macrophage polarization and tumor survival. Dependent on alternative-NFkB signaling, tumor-specific secretion of IL32β led to the corruption of the microenvironment through the polarization of monocytes into specific MCL-associated macrophages, which in turn favor tumor survival. While IL32β-stimulated macrophages secreted several protumoral factors, they supported MCL survival through BAFF and consequent alternative-NFkB activation in tumor cells. Our data shows that targeting IL32b, BAFF or the alternative NFkB pathway through NIK inhibition could also be of major interest for counteracting the multiple cross-talks that occur in the MCL microenvironment and, especially, the CD40L + T-cell / MCL / CD163 + MCL-associated macrophage triad.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal